[最も共有された! √] s p d f orbitals periodic table 710554-What is s p d f orbitals in chemistry
The periodic table consists of four blocks of elements that correspond to s, p, d, and f orbitals being filled After f orbitals come g and h orbitals In theory, if a g block and an h block of elements existed, bow long would the rows of g and h elements be in this theoretical periodic table?Can hold a maximum of 2 electrons;Answer choices 1 3 11 23 s Question 4 SURVEY 30 seconds Q How many orbitals are found in a dsublevel?
Q Is It Possible For An Atomic Orbital To Exist Beyond The S P F And D Orbitals They Taught About In School Like Could There Be A Other Letter Orbital Beyond
What is s p d f orbitals in chemistry
What is s p d f orbitals in chemistry-In the above diagram, the boxes refer to elements and not to orbitals directly For example, the oxygen atom, which contains four electrons in 2p orbitals, is placed in the fourth box in the 2p group If you think about it, this table provides a beautiful view of how the arrangement of the periodic table is a direct consequence of the number of orbitals of each type and their relative energiesAnswer choices 4 5 10 14 s Question 5 SURVEY 30 seconds Q How many


Electron Configurations
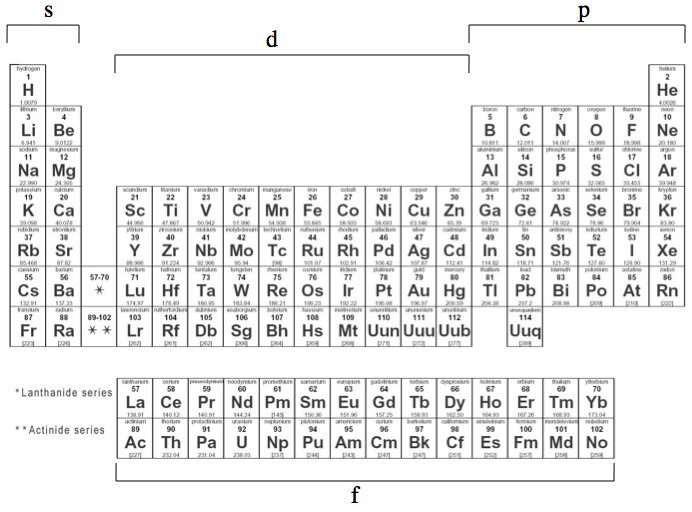
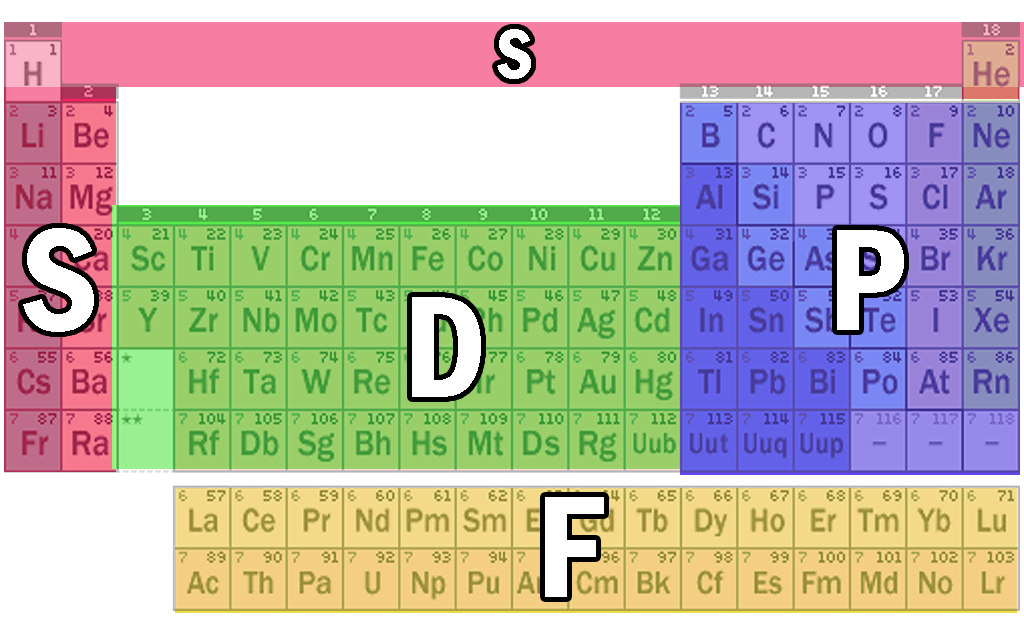
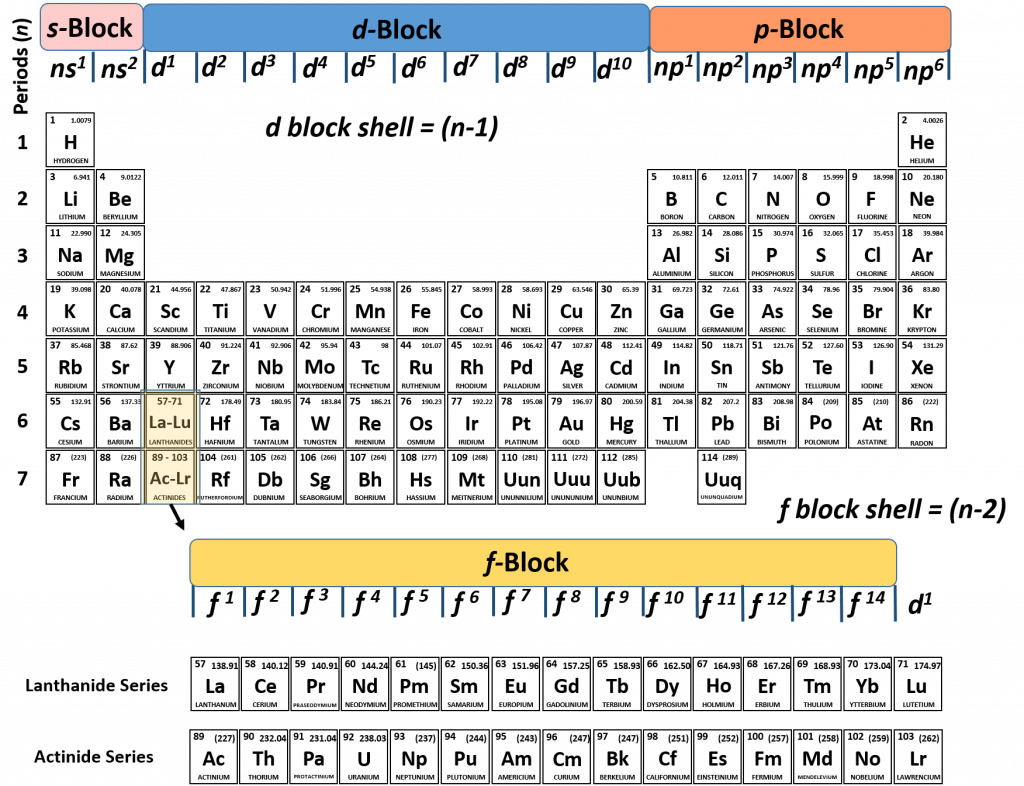
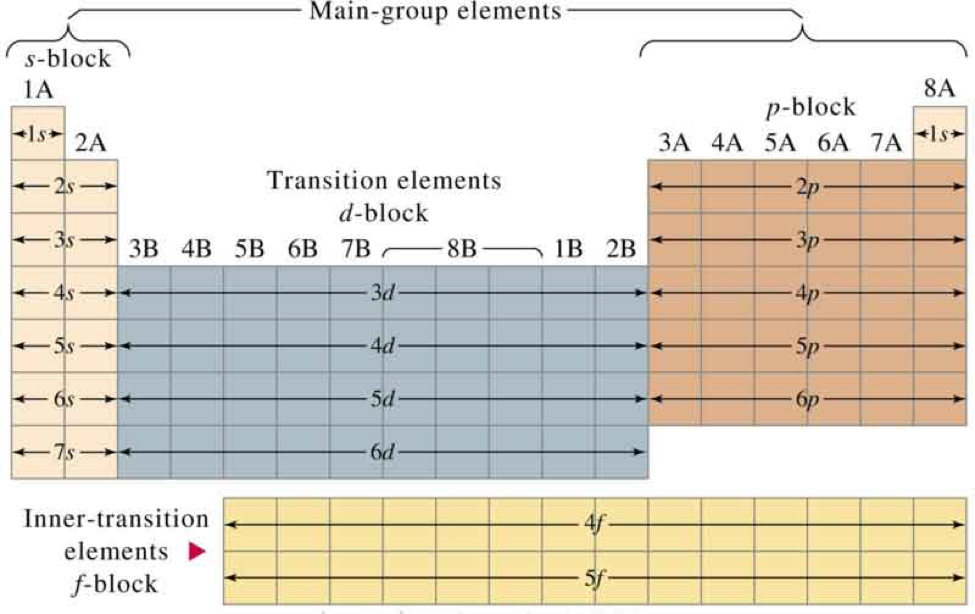
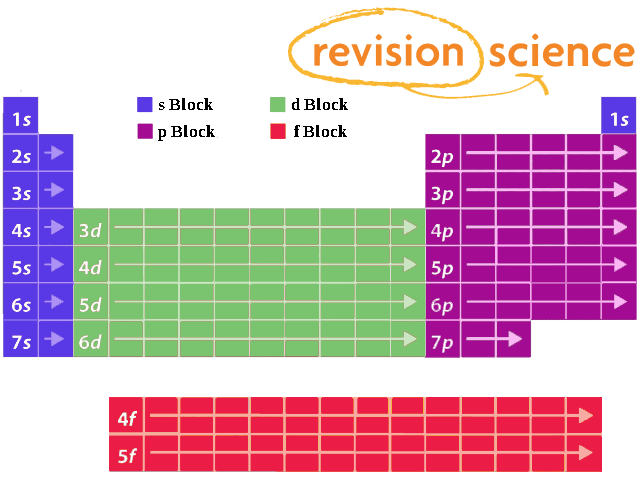
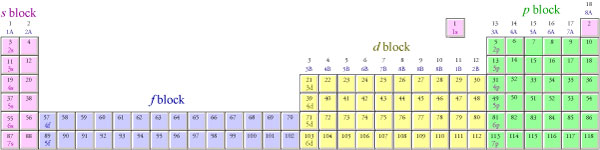
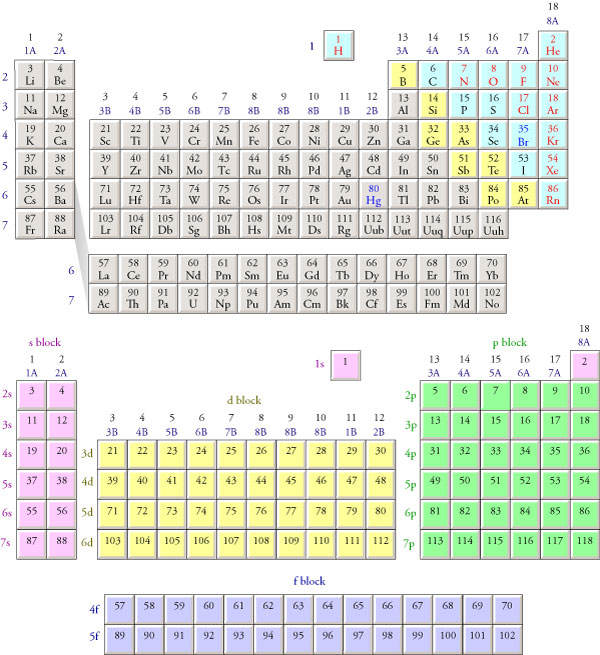
The s, p, d, f and g are called atomic orbitals Filling up these orbitals with electrons builds atoms, and the way in which atoms are build up gives rise to the periodic table There is only one s orbital (m l= 0), but there are three p orbitals (mElements in the long form of periodic table have been divided into four blocks ie s,p,d and f This division is based upon the name of the orbitals which receives the last electronAbout us Periodic Table States Orbitals Electronegativity Evolution Games Learn Calculators Get Help Elements Glossary Contact Boiling Point Melting Help!
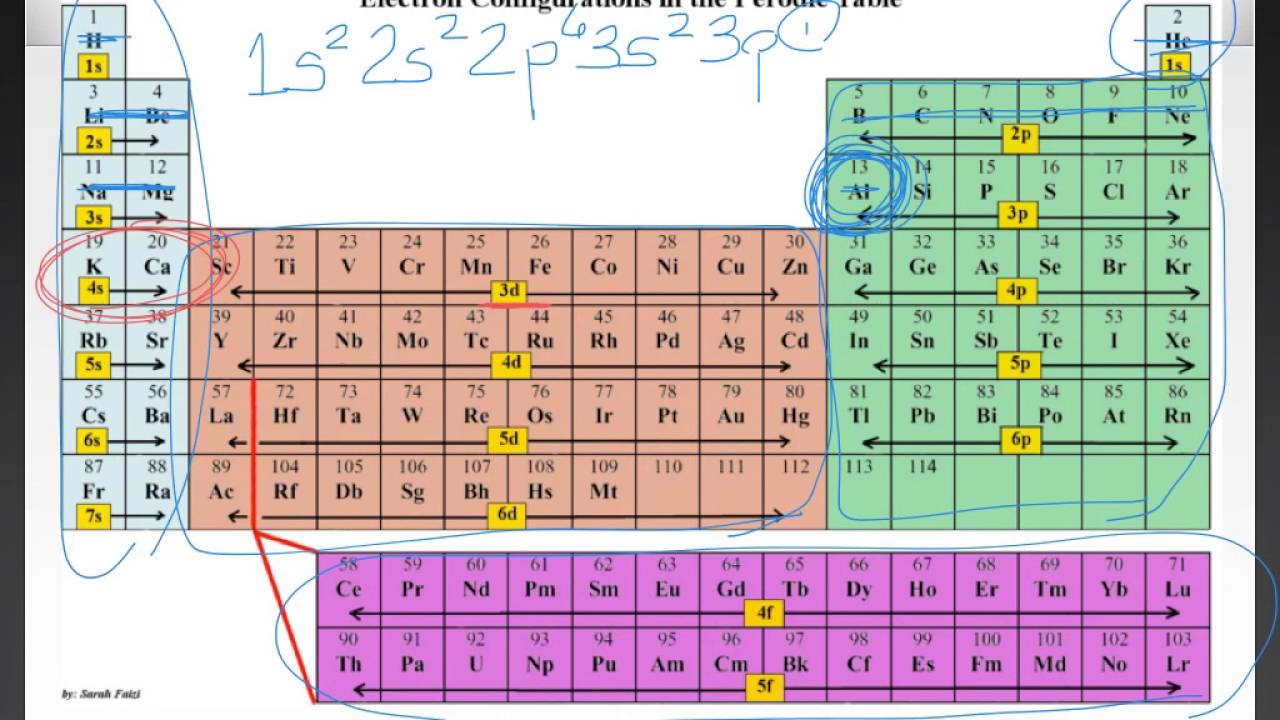
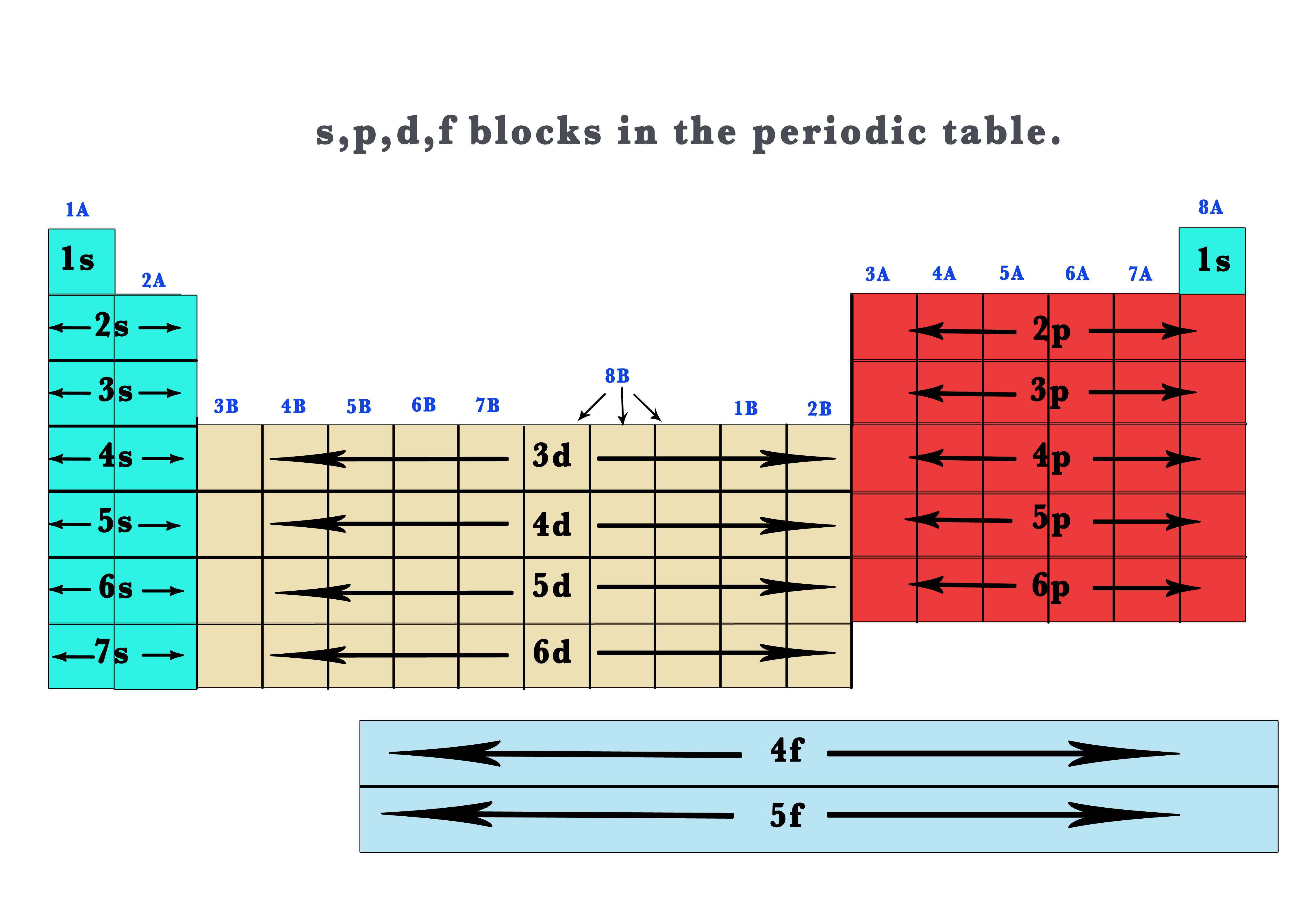
Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled The s , p , d , and f blocks are illustrated below The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occursExplore the Bohr model and atomic orbitals Learn how to use an element's position on the periodic table to predict its properties, electron configuration, and reactivity If you're seeing this message, it means we're having trouble loading external resources on our websiteF ORBITALS At the fourth and higher levels, there are seven f orbitals in addition to the 4s, 4p, and 4d orbitals Counting the 4s, 4p, and 4d orbitals, this makes a total of 16 orbitals in the fourth level They have even more complicated shapes s, p, d, and f orbitals are available at all higher energy levels as well
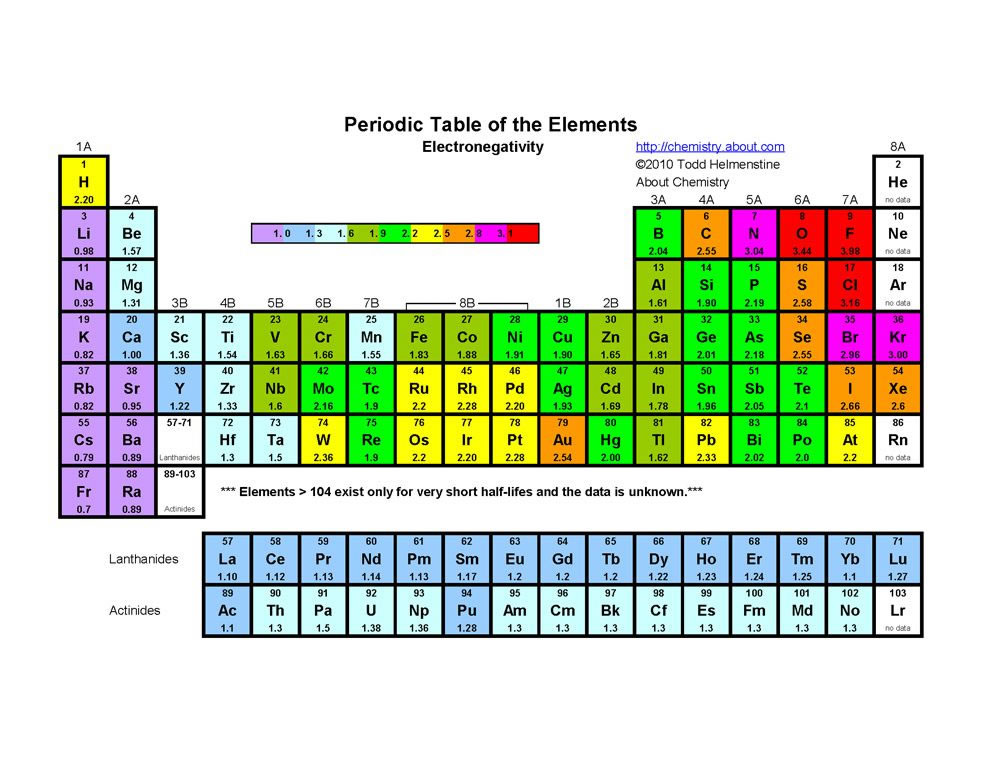
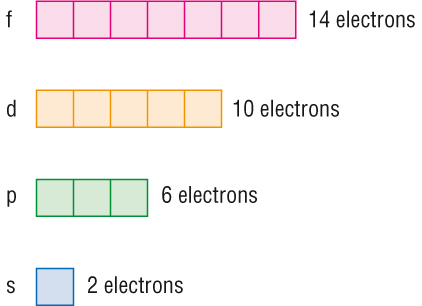
The maximum electrons that can be carried by the subshell S is 2, by P is 6, by D is 10, and the F subshell can carry 14 This decides the electron capacity of the shells The K shell contains a 1s subshell hence it can carry 2 electrons, the L shell has 2s and 2p, and can carry 8 electronsWhile in Moseley's Periodic table, the knowledge of orbitals was much developed and hence we can see s p d and f block on the Moseley's Periodic table Transition elements have the valence electrons in dorbitals, hence they are classified into dblock on Moseley's Periodic tableSodium is found on the third row or Period 3 of the periodic table How many energy levels do the electrons of a sodium atom occupy?


Electron Configurations


The Order Of Filling 3d And 4s Orbitals Chemistry Libretexts
Importance of the s,p,d,f blocks in periodic table Importance of the s,p,d,f blocks in periodic table Anonymous (not verified) Wed, 10/27/10 2305 I need some help with understanding Electron Configuration My teacher tried to help but it wasn't very helpful p block elements have the p orbitals being filled eg N = 1s2 2s2 2p1 2p1 2p1Regions within the energy levels;Location of the four blocks of elements s, p, d, f in the long form periodic table The 's' block elements 's' block elements, (except helium) are those in which the valenceshell is the 'ns' orbital and where the last electron enters into the 's' orbital of the outer element


1


Electron Arrangement In Atoms Youtube
The periodic table consists of four blocks of elements which correspond to the s, p, d, and f orbitals being filled After f orbitals come g and h orbitals In theory, if a g block and h block of elements existed, how long would the rows of g and h elements be in this theoretical periodic table Why?Importance of the s,p,d,f blocks in periodic table Importance of the s,p,d,f blocks in periodic table Anonymous (not verified) Wed, 10/27/10 2305 I need some help with understanding Electron Configuration My teacher tried to help but it wasn't very helpful p block elements have the p orbitals being filled eg N = 1s2 2s2 2p1 2p1 2p1These orbitals are designated as s, p, d, f among othersThe energy incre Snehapareek277 Snehapareek277 Chemistry Secondary School Variations of s p d and f orbital energies of atoms in the periodic table 1 See answer Snehapareek277 is waiting for your help Add your answer and earn points


The Periodic Table Electron Shells And Orbitals Article Khan Academy


Valence Electron Assignment Point
S, p, d, and f The different sections of the Periodic Table are very important in understanding Electron Configuration There are 4 "Blocks" in the Periodic Table the sblock, pblock, dblock, & fblock Remember the special rules for the d and f blocks d – n1 f – n 2The periodic table shows us the sequential filling of the electronsThe energy of the orbitals determines the sequence of filling Lower energy orbitals are always preferred over high energy onesThe table is thus divided into 4 blocks namely – s,p,d, f blocks, depending on the occupation of the respective orbitals by the valence electrons of an elementThe orbital letters are associated with the angular momentum quantum number, which is assigned an integer value from 0 to 3 The s correlates to 0, p to 1, d to 2, and f to 3 The angular momentum quantum number can be used to give the shapes of the electronic orbitals What Does S, P, D, F Stand For?
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)


Electron Configuration Chart


Spdf Orbitals Chart Drone Fest
The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d, and f atomic orbitals, respectively, although for higher values of the quantum number n, particularly when the atom in question bears a positiveIn the case of chromium, an electron from the 4s orbital moves into a 3d orbital, allowing each of the five 3d orbitals to have one electron, making a halffilled set of orbitals In the case of copper, silver and gold, an electron from the highestoccupied s orbital moves into the d orbitals, thus filling the d subshell Many anomalous electron configurations occur in the heavier transitionThe actinides and lanthanides have been placed at the bottom of the periodic table to avoid the undue expansion of the periodic table 1 I st inner transition or 4 fseries, contains 14 elements 58 Ce to 70 Lu Filling of electrons takes place in 4f subshell 2 IInd inner transition or 5 fseries, contains 14 elements 90 Th to 103 Lr Filling



Free Printable Periodic Tables Pdf And Png Science Notes And Projects



Spdf Orbitals Location Diagram Quizlet
2s is lower energy than 2p)(image source)So for example,Number of orbitals differs with sublevel type (s,p,d,f)S, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;


41 The Periodic Table S P D F Blocks Madoverchemistry Com



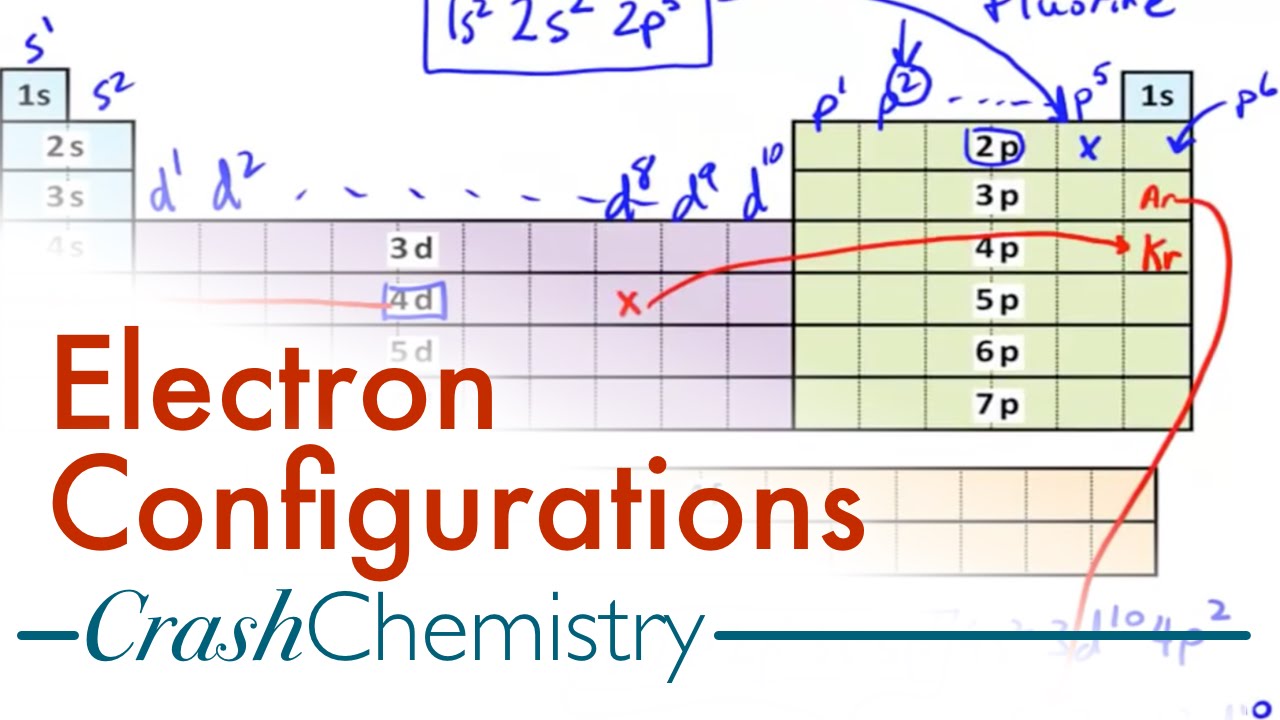
Writing Electron Configurations Using Only The Periodic Table Youtube
2s is lower energy than 2p)(image source)So for example,The four different orbital forms (s, p, d, and f) have different sizes and one orbital will accommodate up to two electrons at most The orbitals p, d, and f have separate sublevels and will thus accommodate more electrons As shown, each element's electron configuration is unique to its position on the periodic tableGroups States Orbitals Electronegativity Evolution Orbitals and Electron Configuration The Periodic Table with Oxidation Numbers and Electron Configurations S Block P Block D



Q Is It Possible For An Atomic Orbital To Exist Beyond The S P F And D Orbitals They Taught About In School Like Could There Be A Other Letter Orbital Beyond


Visualizing Electron Orbitals
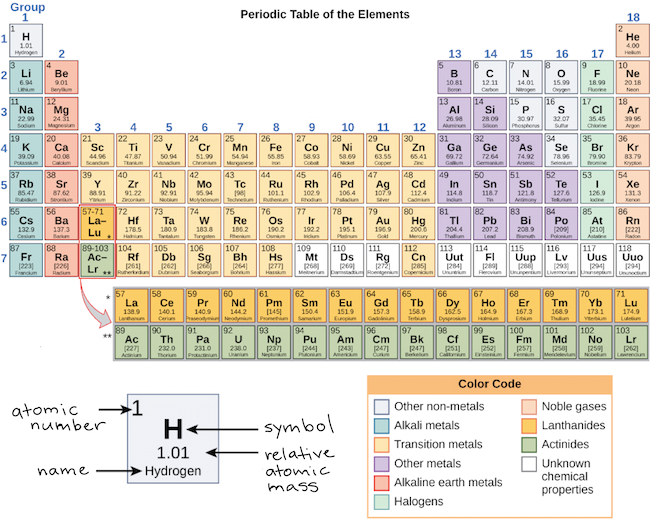
The actinides and lanthanides have been placed at the bottom of the periodic table to avoid the undue expansion of the periodic table 1 I st inner transition or 4 fseries, contains 14 elements 58 Ce to 70 Lu Filling of electrons takes place in 4f subshell 2 IInd inner transition or 5 fseries, contains 14 elements 90 Th to 103 Lr FillingShapes of Orbitals and Electron Density Patterns The s orbitals are spherical, while p orbitals are polar and oriented in particular directions (x, y, and z) It may be simpler to think of these two letters in terms of orbital shapes (d and f aren't described as readily)However, if you look at a crosssection of an orbital, it isn't uniformThe \(s\) sublevel has one orbital, the \(p\) sublevel has three orbitals, the \(d\) sublevel has five orbitals, and the \(f\) sublevel has seven orbitals In the first period, only the \(1s\) sublevel is being filled Since all orbitals can hold two electrons, the entire first period consists of just two elements



The Actinide Research Quarterly 1st Quarter 04


Electron Configuration Tutorial How To Derive Configurations From Periodic Table Crash Chemistry Youtube
Corresponds to the block grouping s,p,d,f on the Periodic Table orbitals regions within electron cloud where electrons orbit the nucleus;While in Moseley's Periodic table, the knowledge of orbitals was much developed and hence we can see s p d and f block on the Moseley's Periodic table Transition elements have the valence electrons in dorbitals, hence they are classified into dblock on Moseley's Periodic tableThe \(s\) sublevel has one orbital, the \(p\) sublevel has three orbitals, the \(d\) sublevel has five orbitals, and the \(f\) sublevel has seven orbitals In the first period, only the \(1s\) sublevel is being filled Since all orbitals can hold two electrons, the entire first period consists of just two elements



Electron Configuration Of Atoms Shortcut Tutorial Video Youtube


Modern Periodic Table S P D F Blocks Periodic Table Timeline
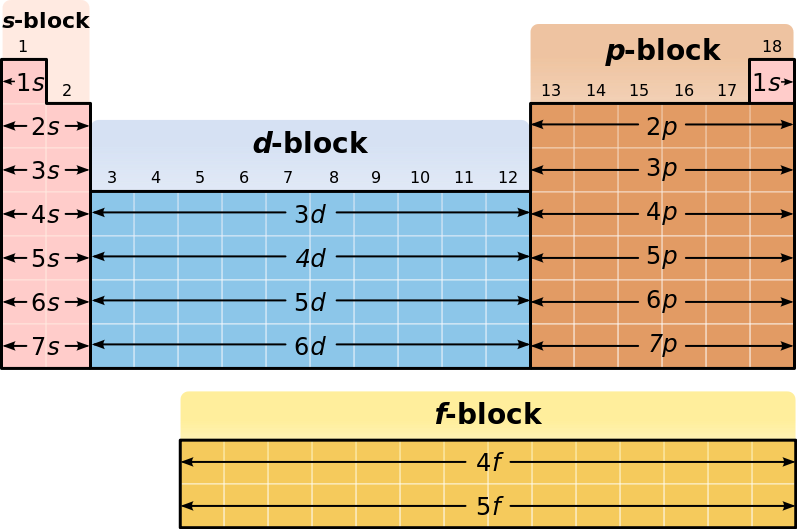
The periodic table consists of four blocks of elements which correspond to the s, p, d, and f orbitals being filled After f orbitals come g and h orbitals In theory, if a g block and h block of elements existed, how long would the rows of g and h elements be in this theoretical periodic table Why?10 being D orbital and 14 being the F orbital your over thinking things All it is basically is the periodic table Look at the periodic table you will see that the S orbitals are in groups 1 and 2 P orbitals are boron noble gas groups D orbitals are transition metals (count from SC to ZN how many elements are there?The s block is 2 groups wide, the p block is 6 groups wide, the d block is 10 groups, and the f block is 14 groups


Electron Configurations



7 3 Electron Configurations Of Atoms Chemistry Libretexts
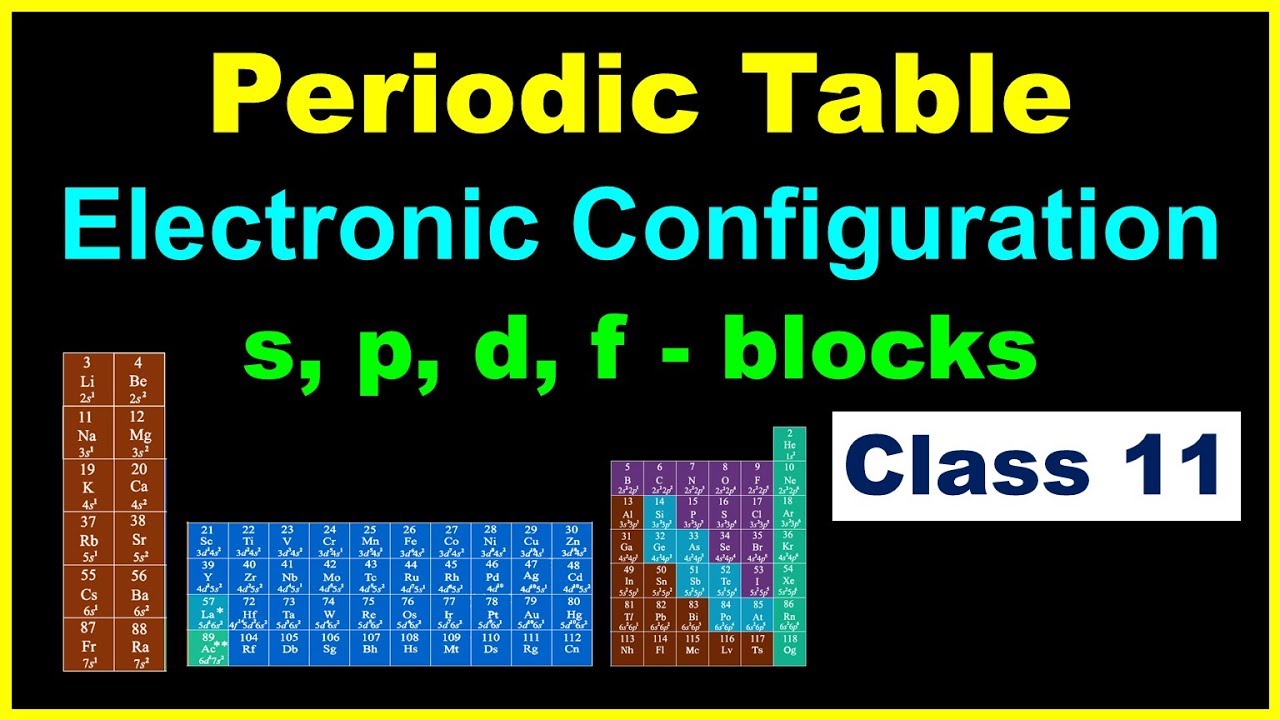
332 Identify the organization of the periodic table into energy levels, sublevels (s, p, d, and f), and orbitals Using the periodic table, write electron configurations for atoms up to Z=18 Identify the repeating pattern of electron configurations for elements in a groupInteractive periodic table showing names, electrons, and oxidation states Visualize trends, 3D orbitals, isotopes, and mix compounds Fully descriptive writeupsPeriodic table 8b why 3 columns Tutorial on s,p,d,f orbitals picture of periodic table with d block what is the 2p block periodic table why there are three 8b elements in periodic table show images of periodic table showing mass and atomic numbers periodic table visual basic pblock elementstutorial "pblock" chemistrytutorial The four main



S Block Elements On The Periodic Table Properties Overview Video Lesson Transcript Study Com


Parsing The Spdf Electron Orbital Model
What is the relationship between the width of the s, p, d, and f blocks on the periodic table and the number of orbitals filled?The elements of the Periodic table are also classified block wise There are 4 blocks in the Periodic table s block, p block, d block and f block The elements which are in the s block have the valence electrons in sorbitals Similarly, elements which are in the p block have the valence electrons in porbitalsThe forbitals start with the lanthanides and the actinides, in the n=6 row and the forbitals have an n=4 as the principle quantum energy number Specifically, the forbitals start with the element 58 n=4 is the energy level, b/c I believe it's in a lower energy state than the dorbitals, which is lower than the sorbitals Hope this helps!


Electronic Configuration And The Periodic Table Table Chemistry For Class 11 In Hindi Youtube



Powerpoint Orbital Shape Orientation Spdf Periodic Table Powerpoint Presentation Free Online Download Ppt 6tz333
The actinides and lanthanides have been placed at the bottom of the periodic table to avoid the undue expansion of the periodic table 1 I st inner transition or 4 fseries, contains 14 elements 58 Ce to 70 Lu Filling of electrons takes place in 4f subshell 2 IInd inner transition or 5 fseries, contains 14 elements 90 Th to 103 Lr FillingThe block names (s, p, d, f) originated from descriptions of spectroscopic lines of atomic orbitals sharp, principal, diffuse, and fundamental No gblock elements have been observed to date, but the letter was chosen because it is next in alphabetical order after fThe periodic table, electron shells, and orbitals Shells, subshells, and orbitals This is the currently selected item Introduction to electron configurations subshell, or sometimes people will say sublevels and that's where they're talking about s or p or d and eventually f so if I circle this, I'm talking about that first shell Now
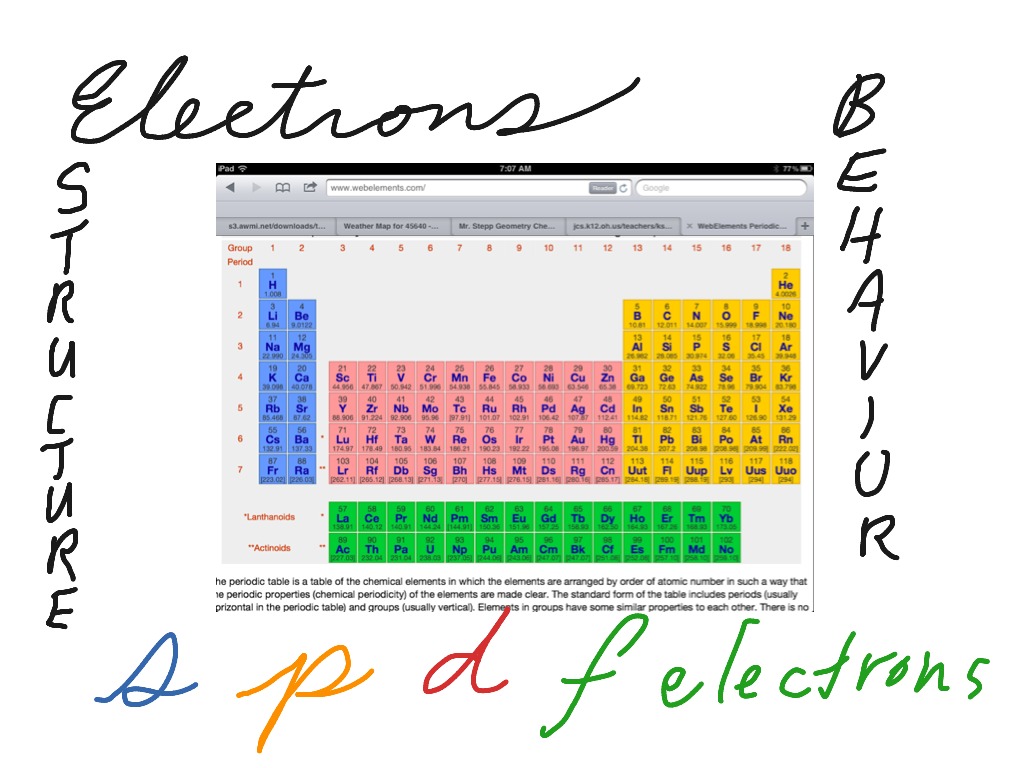


S P D F Electron Blocks On The Periodic Table Chemistry Periodic Table Electron Configuration Showme



3 1 S P D F Periodic Table Kerem S Chemistry Notes Ib
S, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;In the above diagram, the boxes refer to elements and not to orbitals directly For example, the oxygen atom, which contains four electrons in 2p orbitals, is placed in the fourth box in the 2p group If you think about it, this table provides a beautiful view of how the arrangement of the periodic table is a direct consequence of the number of orbitals of each type and their relative energiesThe labels s, p, d and f blocks of the Periodic Table refer to the subshell that is being filled with electrons Group 1 elements occur at the beginning of a new row (Period) of the Periodic Table The highest energy level (valence shell) contains only 1 electron in an s subshell Group 2 elements occur directly to the right of Group 1 elements


1
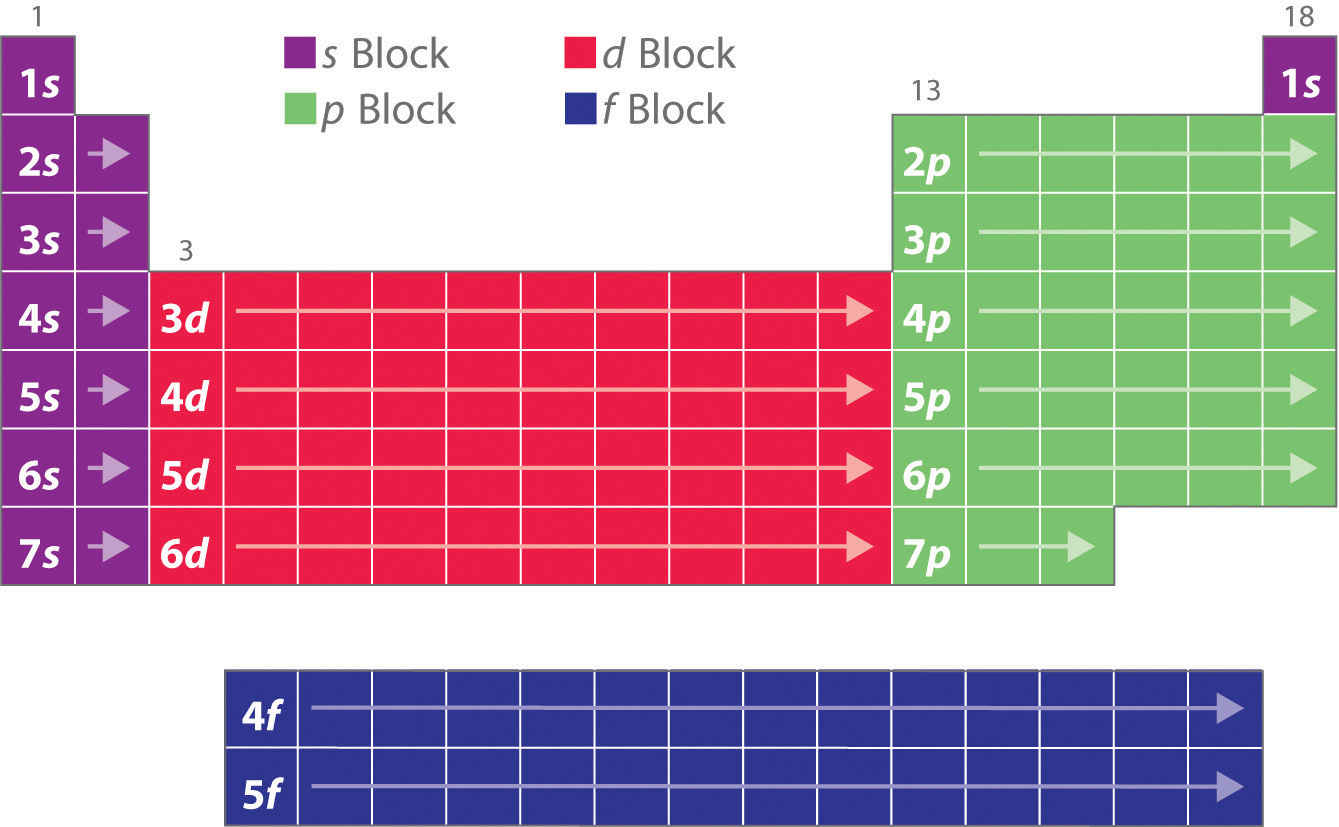


6 9 Electron Configurations And The Periodic Table Chemistry Libretexts
Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled The s , p , d , and f blocks are illustrated below The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occursIn the above diagram, the boxes refer to elements and not to orbitals directly For example, the oxygen atom, which contains four electrons in 2p orbitals, is placed in the fourth box in the 2p group If you think about it, this table provides a beautiful view of how the arrangement of the periodic table is a direct consequence of the number of orbitals of each type and their relative energiesThe \(s\) sublevel has one orbital, the \(p\) sublevel has three orbitals, the \(d\) sublevel has five orbitals, and the \(f\) sublevel has seven orbitals In the first period, only the \(1s\) sublevel is being filled Since all orbitals can hold two electrons, the entire first period consists of just two elements


Module B Lesson 4 1 T Gem And Chemland Mel Burgess And Met



Electronic Configuration Of Elements Trick S P D F Pattern Class 11 Iit Jee Neet Aiims Youtube
The block names (s, p, d, f) originated from descriptions of spectroscopic lines of atomic orbitals sharp, principal, diffuse, and fundamental No gblock elements have been observed to date, but the letter was chosen because it is next in alphabetical order after fPeriodic table 8b why 3 columns Tutorial on s,p,d,f orbitals picture of periodic table with d block what is the 2p block periodic table why there are three 8b elements in periodic table show images of periodic table showing mass and atomic numbers periodic table visual basic pblock elementstutorial "pblock" chemistrytutorial The four mainThe four different orbital forms (s, p, d, and f) have different sizes and one orbital will accommodate up to two electrons at most The orbitals p, d, and f have separate sublevels and will thus accommodate more electrons As shown, each element's electron configuration is unique to its position on the periodic table
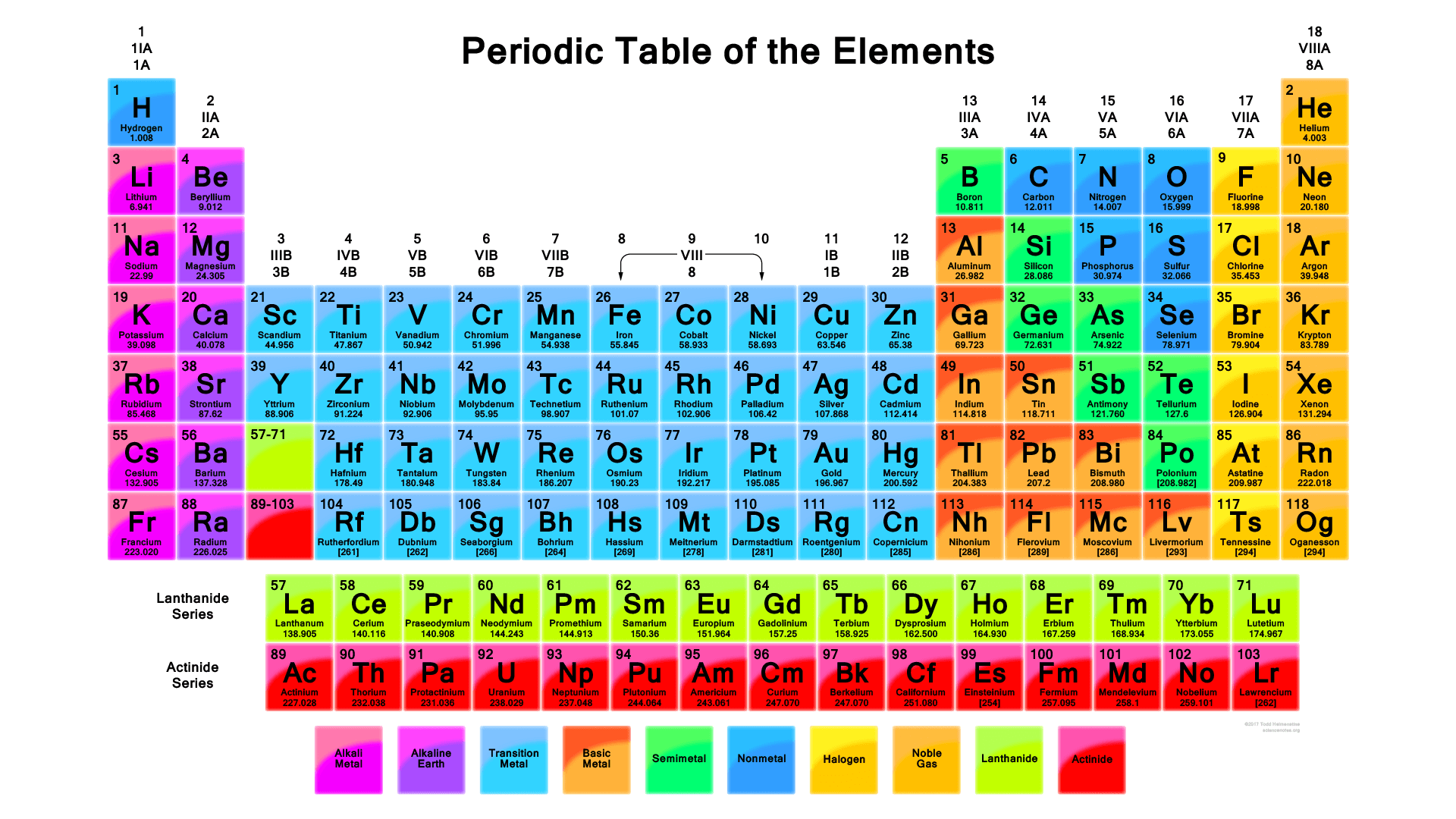


How Do You Determine The Electron Configuration Of W Socratic


Chapter 6 The Periodic Table And Periodic Law Mr Caldwell S Classroom
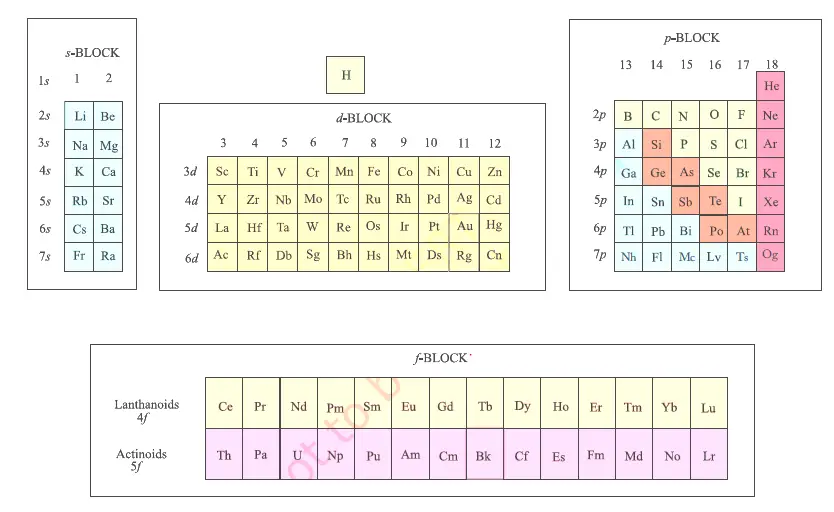


Modern Periodic Table S P D F Blocks Elements


Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes


1
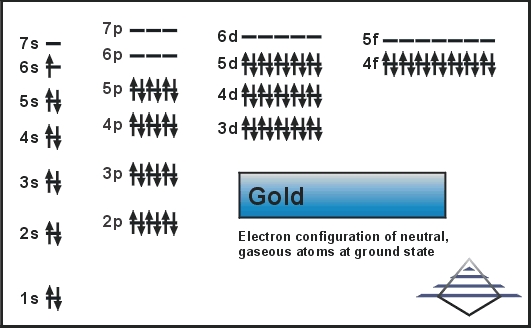


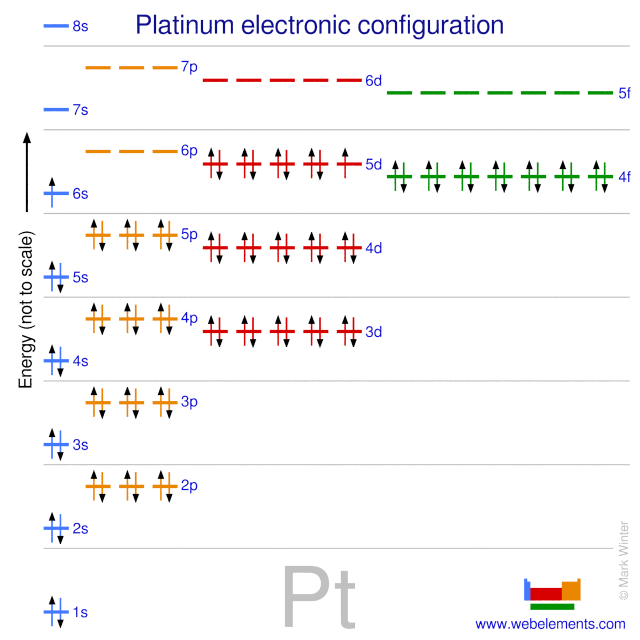
How To Find Elements Electron Configuration For Gold Au



Dublin Schools Lesson Electron Configurations Using The Periodic Table
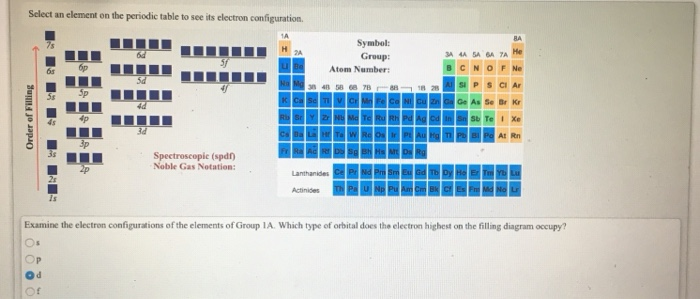


Solved Select An Element On The Periodic Table To See Its Chegg Com
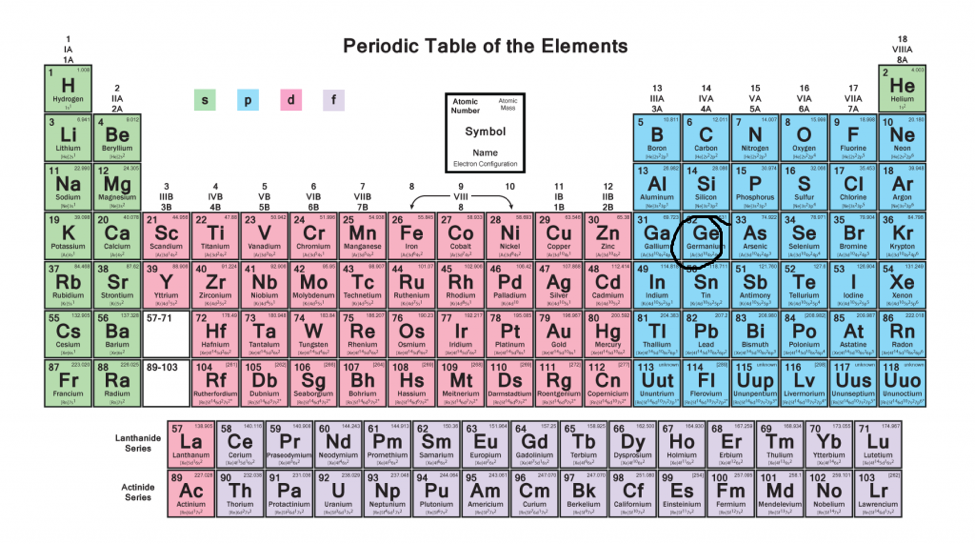


Electron Configurations A Must Know Hack



Ninth Grade Lesson Introduction To Electron Orbital Levels
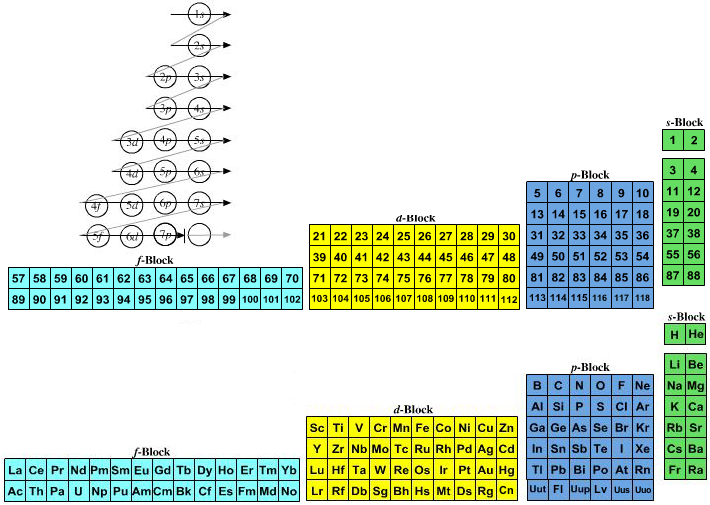


Quantum Numbers To Periodic Tables Chemogenesis



Periodic Table Spdf Blocks Page 1 Line 17qq Com


Ch150 Chapter 2 Atoms And Periodic Table Chemistry


5 6 Atomic Electron Configurations Chemistry Libretexts



Which Orbital Indicated On The Periodic Table S P D F Help Asap I Ll Mark As Brainlister Brainly Com



Valence Electron Definition Configuration Example Study Com Chemistry Lessons Chemistry Classroom Electron Configuration


Electron Configurations A Must Know Hack



Dublin Schools Lesson Electron Configurations Using The Periodic Table


Parsing Spdf Orbital Hybridization And Simple Bonding



What Are Element Blocks On The Periodic Table



The Compound Interest Periodic Table Of Data Compound Interest



File Subshells Of Orbitals Jpg Wikibooks Open Books For An Open World



Periodic Table Spdf Part 2 Ground State Configuration Youtube



Electron Configuration For Calcium Ca


Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes


Parsing The Spdf Electron Orbital Model


Electron Configurations



Periodic Table Download Periodic Table Pdf Groups Element Names List


If Each Orbital Can Hold A Maximum Of 3 Electrons The Number Of Elements In The 4th Period Of The Periodic Table Long Form Is Socratic


Shells And Subshells A Level Chemistry



Ground State Electron Configuration Definition Example Video Lesson Transcript Study Com



Periodic Table Blocks Of Elements



Electron Configuration Boundless Chemistry



Chm122 2 5 11 Spdf Block Elements Youtube
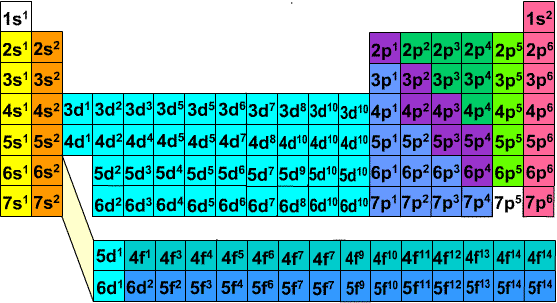


Electron Configurations



Modern Periodic Table Of Elements Periodicity Trends Videos Examples



Electron Configuration For Carbon C


Parsing The Spdf Electron Orbital Model


Ib Chemistry Topic 12 1 Electronic Configuration
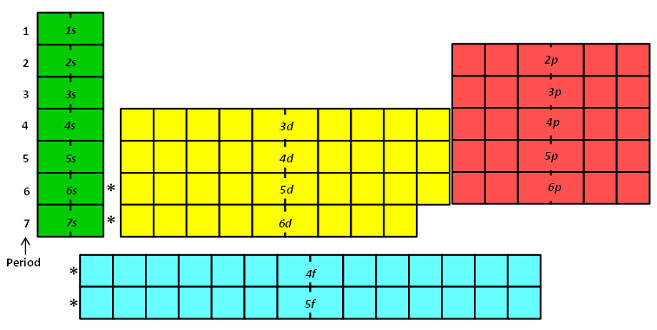


Electron Configuration Texas Gateway



Modern Periodic Table S P D F Blocks Elements



Electron Configurations Atomic Properties And The Periodic Table
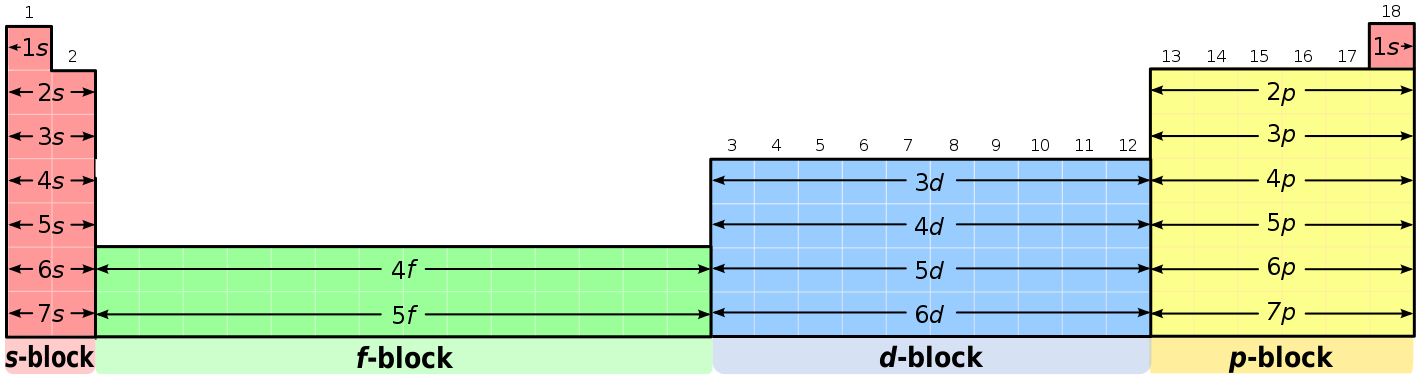


File Periodic Table Blocks Spdf 32 Column Svg Wikimedia Commons



Electronic Configuration Of The D Block Elements Concepts Videos Q As



Oneclass Write The Electron Configurations For P And Cl Using Both Spdf Notation And Orbital Box Dia



Electron Configuration Anomalies Villanova College Chemistry Blog
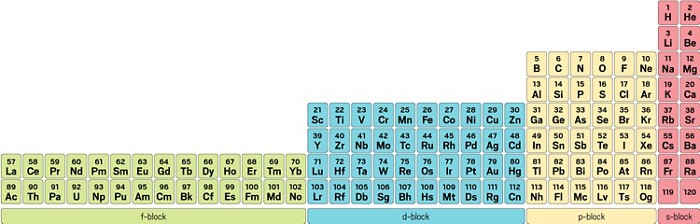


The Periodic Table Is An Icon But Chemists Still Can T Agree On How To Arrange It


What Is The Electron Configuration For Francium Socratic


The Periodic Table Is An Icon But Chemists Still Can T Agree On How To Arrange It


Www Gusd Net Cms Lib Ca Centricity Domain 1907 6the periodic table 3 5web Pdf


Webelements Periodic Table Platinum Properties Of Free Atoms



Periodic Table With Spdf Configuration Of All Elements Periodic Table Electron Configuration Configuration



Blocks Of The Periodic Table Chemistry For Non Majors


Electron Configuration Chemistry 10


Www Ch Ntu Edu Tw Sfcheng Html Genchem05 Chapt6 Brown holme 2 Pdf
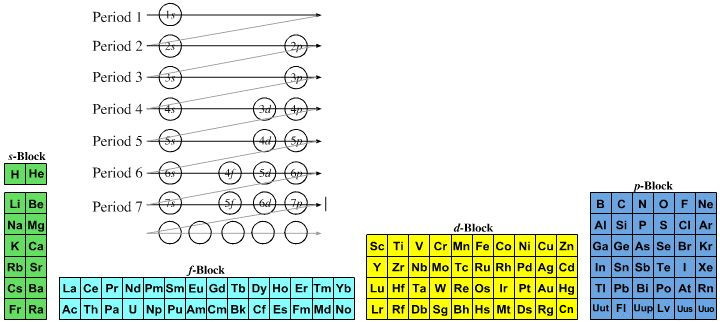


Quantum Numbers To Periodic Tables Chemogenesis
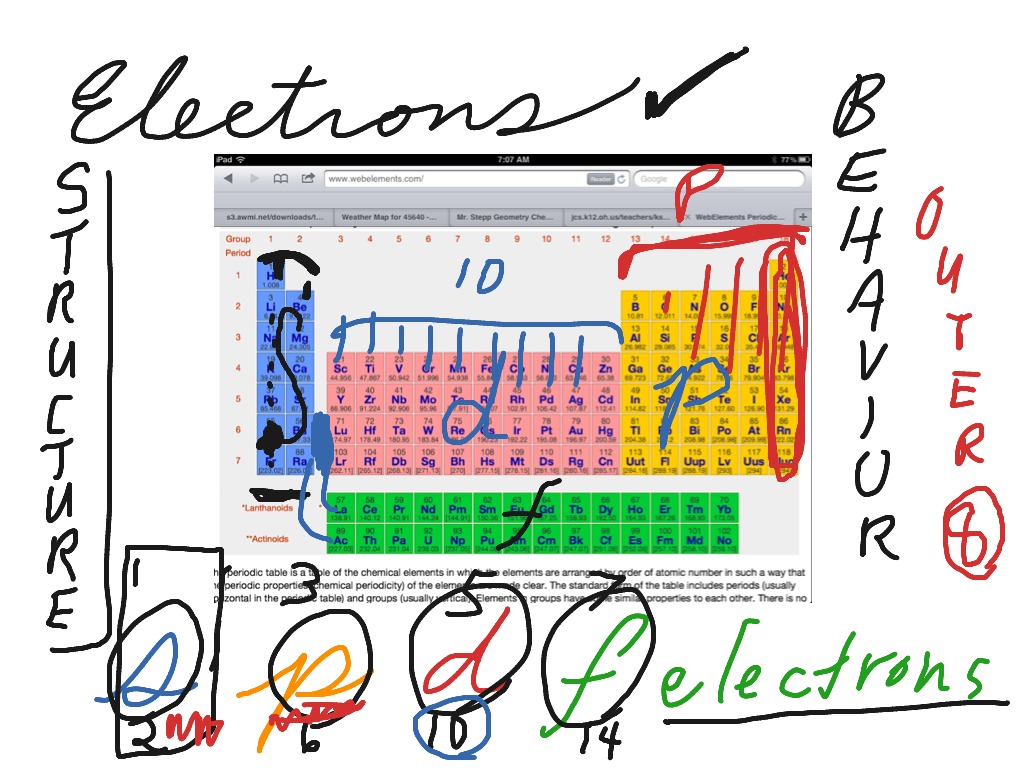


S P D F Electron Blocks On The Periodic Table Chemistry Periodic Table Electron Configuration Showme


Parsing The Spdf Electron Orbital Model
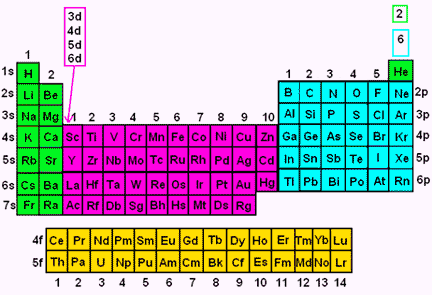


Electron Configuration Wyzant Resources


7 Ways To Color The Periodic Table Students Learn Faster And Retain More
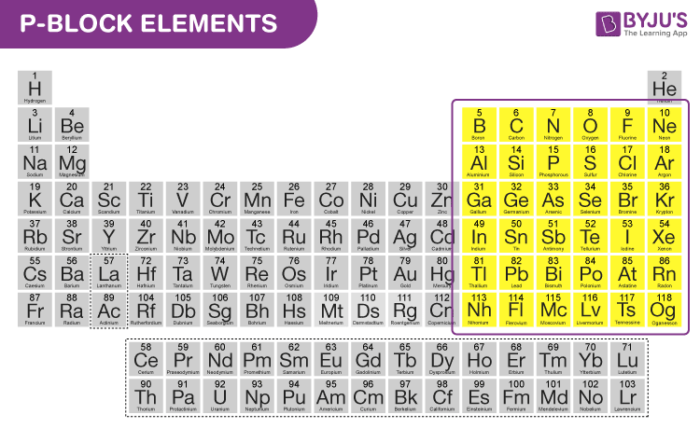


P Block Elements On Periodic Table Introduction Properties Trends



Block Periodic Table Wikipedia


Electron Configurations And The Periodic Table



A Chart Of The Spdf Electron Orbitals Chemistry Education Chemistry Classroom Teaching Chemistry



Electron Configuration Basics Chemistrybytes Com



Ib Chemistry On Quantum Numbers And Electronic Configuration
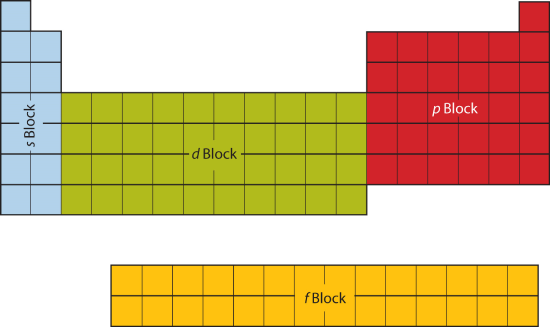


9 7 Electron Configurations And The Periodic Table Chemistry Libretexts
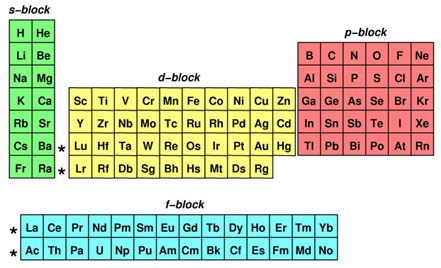


Electron Configuration Texas Gateway


Blocks Of The Periodic Table


1


Electron Configurations
/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)


What Are Element Blocks On The Periodic Table



Periodic Table


Electron Configurations


コメント
コメントを投稿